Mechanistic origin of partial agonism of tetrahydrocannabinol for cannabinoid receptors
BibTeX
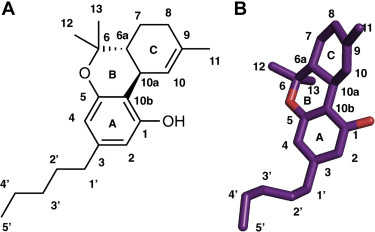
Cannabinoid receptor 1 (CB1) is a therapeutically relevant drug target for controlling pain, obesity, and other central nervous system disorders. However, full agonists and antagonists of CB1 have been reported to cause serious side effects in patients. Therefore, partial agonists have emerged as a viable alternative to full agonists and antagonists as they avoid overstimulation and side effects. One of the key bottlenecks in the design of partial agonists is the lack of understanding of the molecular mechanism of partial agonism. In this study, we examine two mechanistic hypotheses for the origin of partial agonism in cannabinoid receptors and explain the mechanistic basis of partial agonism exhibited by Î9-Tetrahydrocannabinol (THC). In particular, we inspect whether partial agonism emerges from the ability of THC to bind in both agonist and antagonist binding pose or from its ability to only partially activate the receptor. Extensive molecular dynamics simulations and the Markov state model capture the THC binding in both antagonist, and agonist binding poses in CB1 receptor. Furthermore, we observe that binding of THC in the agonist binding pose leads to rotation of toggle switch residues and causes partial outward movement of intracellular transmembrane helix 6 (TM6). Our simulations also suggest that the alkyl side chain of THC plays a crucial role in determining partial agonism by stabilizing the ligand in the agonist and antagonist-like poses within the pocket. This study provides us fundamental insights into the mechanistic origin of the partial agonism of THC.

