Structural Rearrangement of the Serotonin Transporter Intracellular Gate Induced by Thr276 Phosphorylation
BibTeX
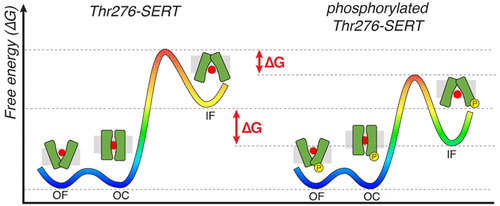
The reuptake of the neurotransmitter serotonin from the synaptic cleft by the serotonin transporter, SERT, is essential for proper neurological signaling. Biochemical studies have shown Thr276 of transmembrane helix 5 is a site of PKG-mediated SERT phosphorylation, which has been proposed to shifts the SERT conformational equlibira to promote inward-facing states, thus enhancing 5HT transport. Recent structural and simulation studies have provided insights into the conformation transitions during substrate transport but have not shed light on SERT regulation via post-translational modifications. Using molecular dynamics simulations and Markov state models, we investigate how Thr276 phosphorylation impacts the SERT mechanism and its role in enhancing transporter stability and function. Our simulations show that Thr276 phosphorylation alters the hydrogen-bonding network involving residues on transmembrane helix 5. This in turn decreases the free energy barriers for SERT to transition to the inward-facing state, thus facilitating 5HT transport. The results provide atomistic insights into in vivo SERT regulation and can be extended to other pharmacologically important transporters in the solute carrier superfamily.

