Effect of Histidine Covalent Modification on Strigolactone Receptor Activation and Selectivity.
BibTeX
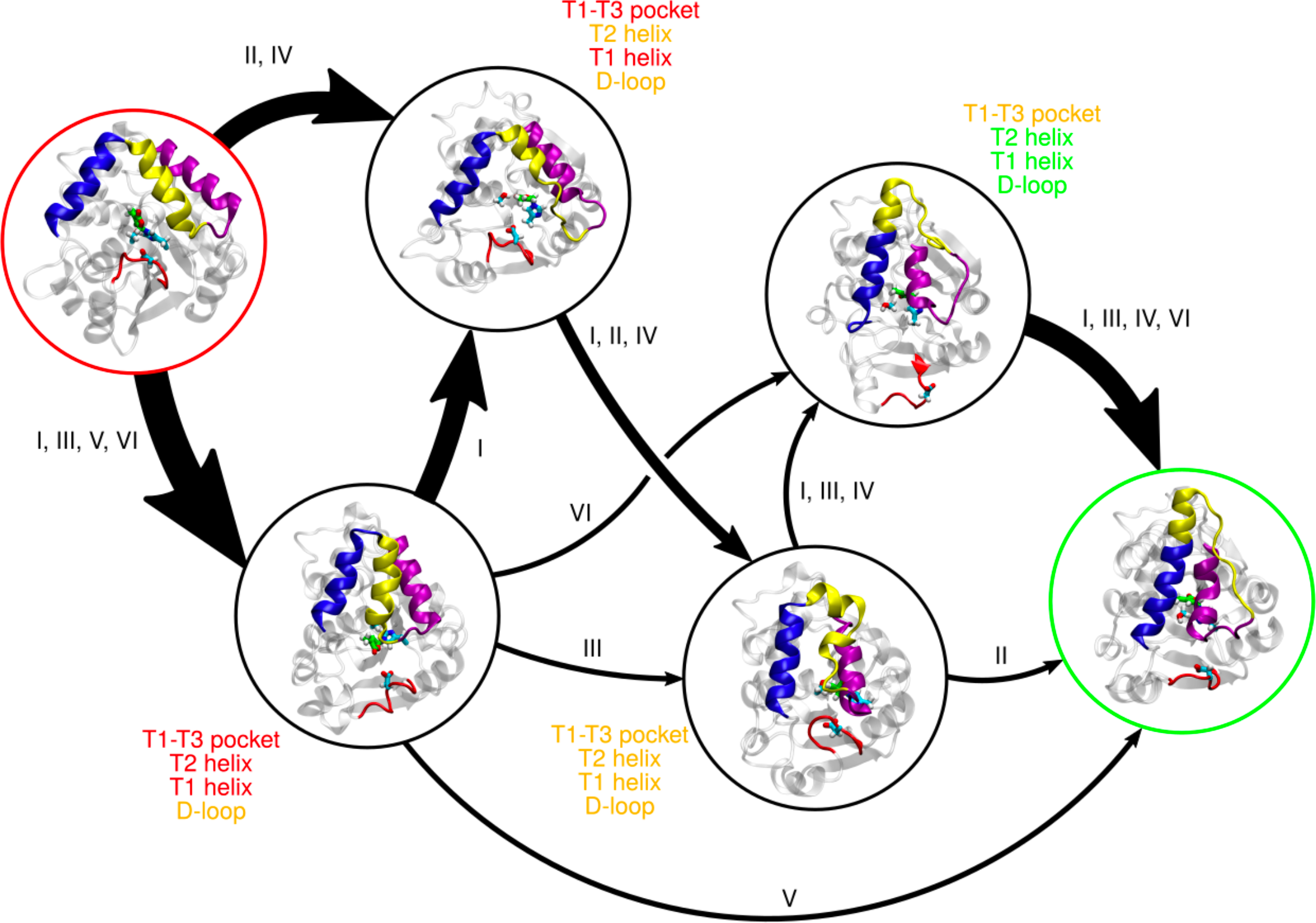
The parasitic weed Striga has led to billions of dollars’ worth of agricultural productivity loss worldwide. Striga detects host plants using the plant hormone strigolactone. Early steps in the strigolactone signaling pathway involve substrate binding and hydrolysis followed by a conformational change to an “active” or “closed” state, after which it associates with a MAX2-family downstream signaling partner. The structures of the inactive and active states of strigolactone receptors are known through X-ray crystallography, and the transition pathway of from the inactive to active state in apo receptors has previously been characterized using molecular dynamics simulations. However, it also has been suggested that a covalent butenolide modification of the receptor on the catalytic histidine through substrate hydrolysis promotes formation of the active state. Using molecular dynamics simulations, we show that the presence of the covalent butenolide enhances activation in both At D14 and ShHTL7, but the enhancement is ∼50 times greater in ShHTL7. We also show that several conserved interactions with the covalent butenolide modification promote transition to the active state in both At D14 (non-parasite) and ShHTL7 (parasite). Finally, we demonstrate that the enhanced activation of ShHTL7 likely results from disruption of ShHTL7-specific histidine interactions that inhibited activation in the apo case.

