Mechanistic Basis for Enhanced Strigolactone Sensitivity in KAI2 Triple Mutant
BibTeX
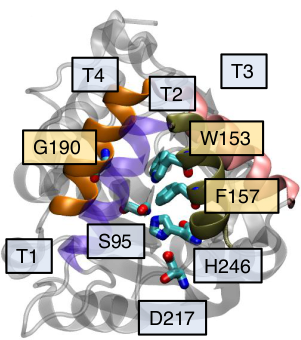
Striga hermonthica is a parasitic weed that destroys billions of dollars’ worth of staple crops every year. Its rapid proliferation stems from an enhanced ability to metabolize strigolactones (SLs), plant hormones that direct root branching and shoot growth. Striga’s SL receptor, ShHTL7, bears more similarity to the staple crop karrikin receptor KAI2 than to SL receptor D14, though KAI2 variants in plants like Arabidopsis thaliana show minimal SL sensitivity. Recently, studies have indicated that a small number of point mutations to HTL7 residues can confer SL sensitivity to AtKAI2. Here, we analyze both wild-type AtKAI2 and SL-sensitive mutant Var64 through all-atom, long-timescale molecular dynamics simulations to determine the effects of these mutations on receptor function at a molecular level. We demonstrate that the mutations stabilize SL binding by about 2 kcal/mol. They also result in a doubling of the average pocket volume, and eliminate the dependence of binding on certain pocket conformational arrangements. While the probability of certain non-binding SL-receptor interactions increases in the mutant compared with the wild-type, the rate of binding also increases by a factor of ten. All these changes account for the increased SL sensitivity in mutant KAI2, and suggest mechanisms for increasing functionality of host crop SL receptors.

