Imparting selective fluorophilic interactions in redox copolymers for the electrochemically mediated capture of short-chain perfluoroalkyl substances
BibTeX
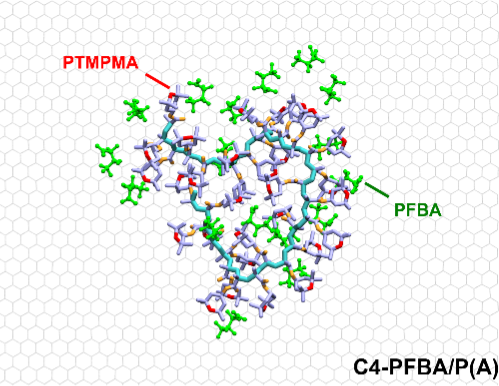
With increasing regulations on per- and polyfluoroalkyl substances (PFAS) across the world, understanding the molecular level interactions that drive their binding by functional adsorbent materials is key to effective PFAS removal from water streams. With the phaseout of legacy long-chain PFAS, the emergence of short-chain PFAS has posed a significant challenge for materials design, due to their higher mobility and hydrophilicity and inefficient removal by conventional treatment methods. Here, we demonstrate how cooperative molecular interactions are essential to target short-chain PFAS (from C4 – C7), by tailoring structural units to enhance affinity while modulating electrochemical control of capture and release of PFAS. We report a new class of fluorinated, redox-active amine-functionalized copolymers to leverage both fluorophilic and electrostatic interactions for short-chain PFAS binding. We combine molecular dynamics (MD) simulations and electrosorption to elucidate the role of the designer functional groups in enabling affinity towards short-chain PFAS. Preferential interaction coefficients from MD simulations correlated closely with experimental trends: fluorination enhanced overall PFAS uptake and promoted capture of less hydrophobic short-chain PFAS (C ≤ 5), while electrostatic interactions provided by secondary amine groups were sufficient to capture PFAS with higher hydrophobicity (C ≥ 6). Addition of an induced electric field showed favorable kinetic enhancement for the shortest PFAS, while increasing the reversibility of release from the electrode. Integration of these copolymers with electrochemical separations showed potential for removing these contaminants at environmentally relevant conditions while eliminating the need for chemical regeneration.

