Activation Mechanism of the Human Smoothened Receptor
BibTeX
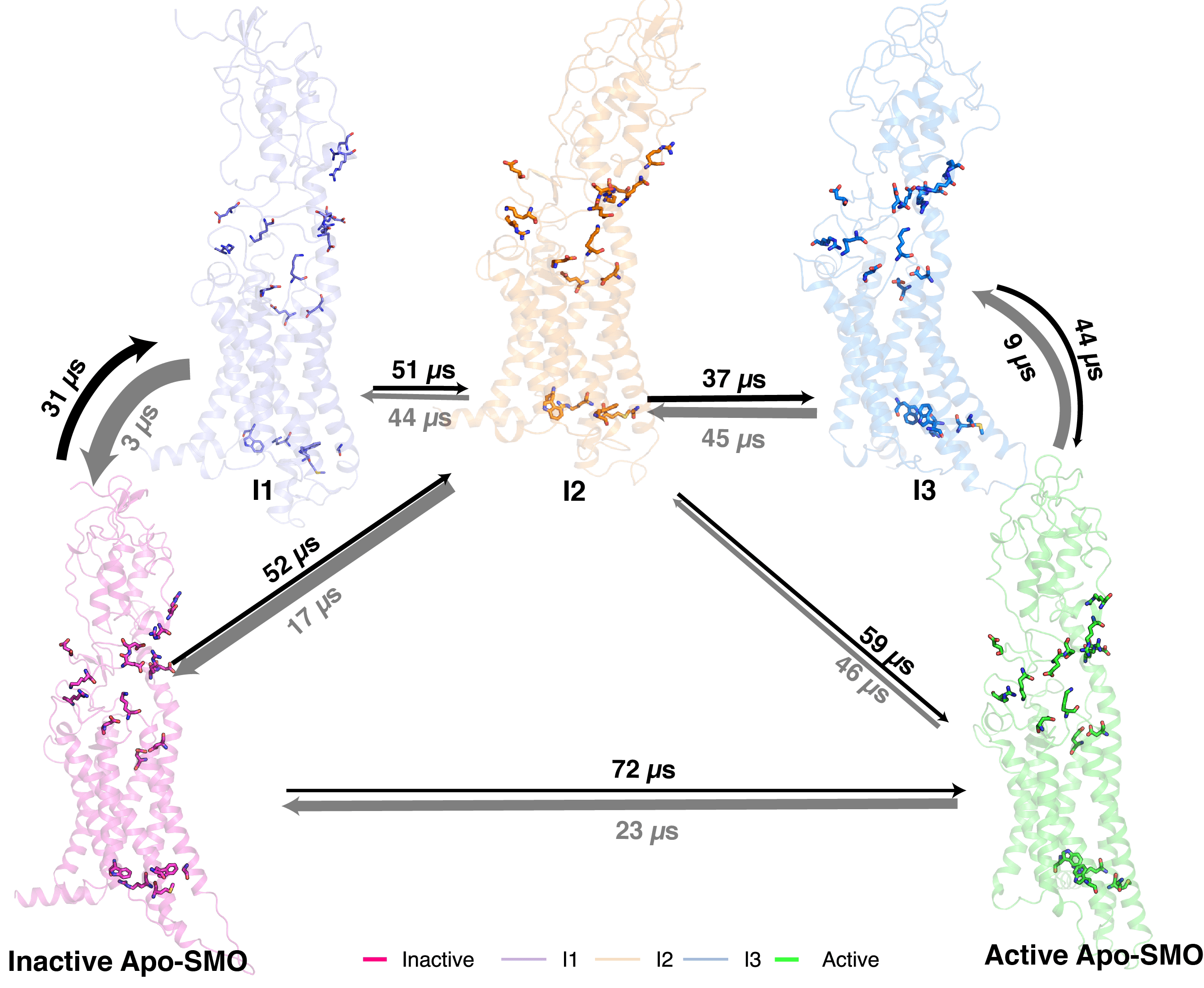
Smoothened (SMO) is a membrane protein of the Class F subfamily of G-Protein Coupled Receptors (GPCRs) and maintains homeostasis of cellular differentiation. SMO undergoes conformational change during activation, transmitting the signal across the membrane, making it amenable to bind to its intracellular signaling partner. Receptor activation has been studied at length for Class A receptors, but the mechanism of Class F receptor activation remain unknown. Agonists and antagonists bound to SMO at sites in the Transmembrane Domain (TMD) and the Cysteine Rich Domain have been characterized, giving a static view of the various conformations SMO adopts. While the structures of the inactive and active SMO outline the residue-level transitions, a kinetic view of the overall activation process remains unexplored for Class F receptors. We describe SMO’s activation process in atomistic detail by performing 300 μs of molecular dynamics simulations and combining it with Markov state model theory. A molecular switch, conserved across Class F and analogous to the activation-mediating D-R-Y motif in Class A receptors, is observed to break during activation. We also show that this transition occurs in a stage-wise movement of the transmembrane helices – TM6 first, followed by TM5. To see how modulators affect SMO activity, we simulated agonist and antagonist-bound SMO. We observed that agonist-bound SMO has an expanded hydrophobic tunnel in SMO’s core TMD, while antagonist-bound SMO shrinks this tunnel, further supporting the hypothesis that cholesterol travels through a tunnel inside Smoothened to activate it. In summary, this study elucidates the distinct activation mechanism of Class F GPCRs and shows that SMO’s activation process rearranges the core transmembrane domain to open a hydrophobic conduit for cholesterol transport.

