Distinct Binding Mechanisms for Allosteric Sodium Ion In Cannabinoid Receptors
BibTeX
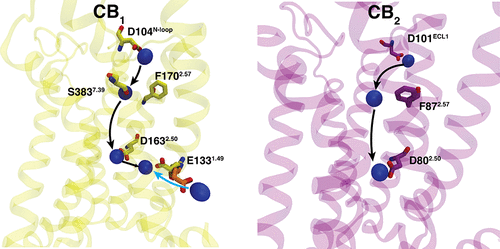
The therapeutical potential of Cannabinoid receptors is not fully explored due to psychoactive side-effects and lack of selectivity associated with the orthosteric ligands. Allosteric modulators have the potential to become selective therapeutics for cannabinoid receptors. Biochemical experiments have shown the effects of the allosteric Na+ binding on cannabinoid receptor activity. However, the Na+ coordination site, and binding pathway are still unknown. Here, we perform molecular dynamic simulations to explore Na+ binding in the cannabinoid receptors, CB1 and CB2. Simulations reveal that Na+ binds to the primary binding site from different extracellular sites for CB1 and CB2. A distinct secondary Na+ coordinate site is identified that is not present in CB2. Furthermore, simulations also show that intracellular Na+ could bind to the Na+ binding site in CB1. Constructed Markov state models show that the standard free energy of Na+ binding is similar to the previously calculated free energy for other class A GPCRs.

